SCREE
Last updated: 2023-06-03
Checks: 7 0
Knit directory: SCREE/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20210907) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version dc5b2b2. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Untracked files:
Untracked: data/workflow.png
Untracked: img/
Untracked: output/workflow.png
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCREE.Rmd) and HTML (docs/SCREE.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| html | 2c16336 | HailinWei98 | 2021-09-23 | Build site. |
| Rmd | a2a5b0a | HailinWei98 | 2021-09-23 | Publish the initial files for myproject |
| html | eeeebf3 | HailinWei98 | 2021-09-23 | Build site. |
| Rmd | 94450d1 | HailinWei98 | 2021-09-23 | Publish the initial files for myproject |
| html | 94450d1 | HailinWei98 | 2021-09-23 | Publish the initial files for myproject |
| html | 518a5ca | HailinWei98 | 2021-09-22 | Build site. |
| Rmd | ab04ccf | HailinWei98 | 2021-09-22 | Publish the initial files for myproject |
| html | 855bd74 | HailinWei98 | 2021-09-22 | Build site. |
| Rmd | e582e5c | HailinWei98 | 2021-09-22 | Publish the initial files for myproject |
SCREE(Single-cell CRISPR scREen data analyses and pErturbation modeliNg) is a comprehensive pipeline to visualize data quality and model perturbation effect of single-cell CRISPR screens RNA-seq/ATAC-seq datasets. SCREE has integrated three R functions: scMAGeCK_lr, Mixscape and plot function of cicero. These functions are used to model the regulatory score between perturbations and genes, estimate the perturbation efficiency of each perturbation and visualize enhancer potential targets, respectively.
Schema
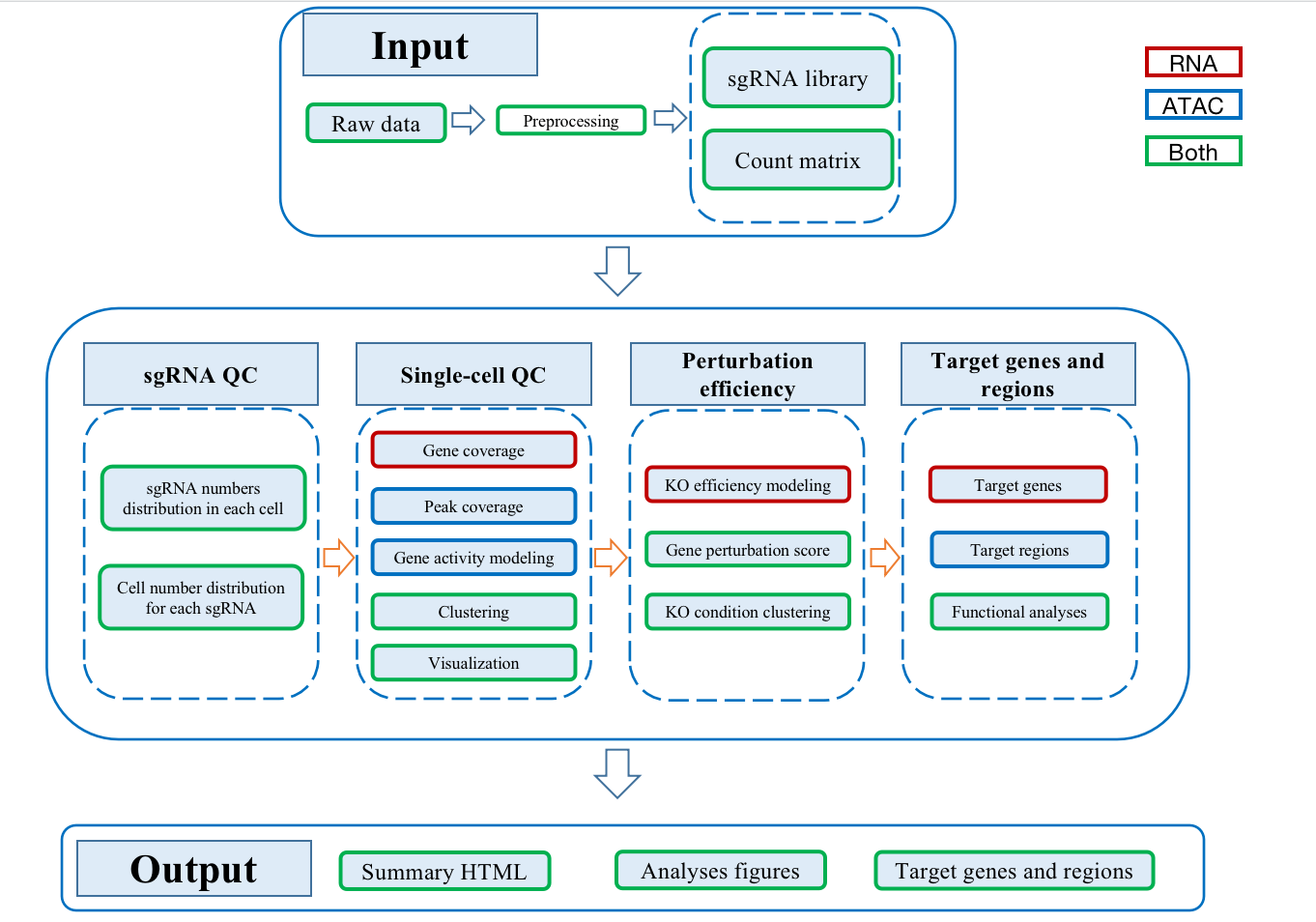
Usage
SCREE provide two major functions, alignment and analysis. To get a full list of commands and descriptions of the alignment part:
SCREE
usage: SCREE [--help/-h][--type/-t][--fasta/-f][--gtf/-g][--10XreferenceConfig/-r][--10Xcsv/-c][--input/-i][--output/-o][--localcores][--localmem]
| Options | Description |
|---|---|
--help/-h |
Show this help message and exit. |
--type/-t |
Input data type, can be one of RNA/ATAC, only support scATAC-seq or scRNA-seq. |
--fasta/-f |
File path of reference in FASTA format. For 10X-like input, this parameter can be omitted. |
--gtf/-g |
File path of reference in gtf format. For 10X-like input, this parameter can be omitted. |
--10Xreference/-r |
File path of config csv file which include reference and library information. Only used for 10X-like input. |
--10Xcsv/-c |
File path of sgRNA reference in csv format. Only used for 10X-like input. |
--input/-i |
File path of fastq files with correct names, required by cellranger. |
--output/-o |
Directory name of output files. |
--localcores |
Set max cores the pipeline may request at one time. Only applies to local jobs. |
--localmem |
Set max GB the pipeline may request at one time. Only applies to local jobs. |
10X config file
The latest version of cellranger can be used to align single-cell CRISPR screens RNA-seq data, which need a config file and a sgRNA reference file as input. More details of the config file can be found in Cellranger multi. Here is the example of config file:
[gene-expression]
reference,/path/to/references/refdata-gex-GRCh38-2020-A
expect-cells,5000
[feature]
reference,/path/to/feature_refs/SC3P_CellPlex_Set_A_millipore_pool_v2_jul_2020.csv
[libraries]
fastq_id,fastqs,lanes,physical_library_id,feature_types,subsample_rate
SC3_v3_NextGem_DI_CRISPR_A549_5K_gex,/path/to/fastqs/SC3_v3_NextGem_DI_CRISPR_A549_5K/SC3_v3_NextGem_DI_CRISPR_A549_5K_gex,any,CRISPR_A549_5K_gex,gene expression,
SC3_v3_NextGem_DI_CRISPR_A549_5K_crispr,/path/to/fastqs/SC3_v3_NextGem_DI_CRISPR_A549_5K/SC3_v3_NextGem_DI_CRISPR_A549_5K_crispr,any,CRISPR_A549_5K_crispr,Crispr Guide Capture,
10X sgRNA reference in csv format
sgRNA reference file is used to do sgRNA alignment. Besides sgRNA names and corresponding target genes, sgRNA reference need the sequence of sgRNA constant region, the sequence next to the spacer sequence in fastq file. Here is the example of sgRNA reference file:
id,name,read,pattern,sequence,feature_type,target_gene_id,target_gene_name
Non-Targeting-5,Non-Targeting-5,R2,(BC)GTTTAAGAGCTAAGCTGGAA,ACTCGAAATCACCTATGGTA,CRISPR Guide Capture,Non-Targeting,Non-Targeting
Non-Targeting-7,Non-Targeting-7,R2,(BC)GTTTAAGAGCTAAGCTGGAA,TTATGTGAGCACGCCATTAC,CRISPR Guide Capture,Non-Targeting,Non-Targeting
Non-Targeting-8,Non-Targeting-8,R2,(BC)GTTTAAGAGCTAAGCTGGAA,CGACGGTAATGCACCTACTA,CRISPR Guide Capture,Non-Targeting,Non-Targeting
APH1A-1,APH1A-1,R2,(BC)GTTTAAGAGCTAAGCTGGAA,GGCAACGCGACCCCACGAG,CRISPR Guide Capture,ENSG00000117362,APH1A
APH1A-2,APH1A-2,R2,(BC)GTTTAAGAGCTAAGCTGGAA,ATGTCACCCCCAGACCCCG,CRISPR Guide Capture,ENSG00000117362,APH1A
CDKN3-1,CDKN3-1,R2,(BC)GTTTAAGAGCTAAGCTGGAA,TGCAGCGCCGGCGACTCAC,CRISPR Guide Capture,ENSG00000100526,CDKN3
CDKN3-2,CDKN3-2,R2,(BC)GTTTAAGAGCTAAGCTGGAA,CGGGGCACCGGTGAGTCGC,CRISPR Guide Capture,ENSG00000100526,CDKN3
EZR-1,EZR-1,R2,(BC)GTTTAAGAGCTAAGCTGGAA,CACTCGGCGGACGCAAGGG,CRISPR Guide Capture,ENSG00000092820,EZR
EZR-2,EZR-2,R2,(BC)GTTTAAGAGCTAAGCTGGAA,GCGCACTCGGCGGACGCAA,CRISPR Guide Capture,ENSG00000092820,EZR
GRB2-1,GRB2-1,R2,(BC)GTTTAAGAGCTAAGCTGGAA,TGCTGCTTCGGCGACCGGG,CRISPR Guide Capture,ENSG00000177885,GRB2
GRB2-2,GRB2-2,R2,(BC)GTTTAAGAGCTAAGCTGGAA,TTCTCGCGGGACACCGACG,CRISPR Guide Capture,ENSG00000177885,GRB2
GSK3A-1,GSK3A-1,R2,(BC)GTTTAAGAGCTAAGCTGGAA,AGCCCAAGCCAGAGCGGCG,CRISPR Guide Capture,ENSG00000105723,GSK3A
GSK3A-2,GSK3A-2,R2,(BC)GTTTAAGAGCTAAGCTGGAA,GAGCGGCGCGGCCTGGAAG,CRISPR Guide Capture,ENSG00000105723,GSK3A
HRAS-1,HRAS-1,R2,(BC)GTTTAAGAGCTAAGCTGGAA,ACCCGAGCCGCACCCGCCG,CRISPR Guide Capture,ENSG00000174775,HRAS
HRAS-2,HRAS-2,R2,(BC)GTTTAAGAGCTAAGCTGGAA,GCACGGGCGGCGGAGACTC,CRISPR Guide Capture,ENSG00000174775,HRAS
JUN-1,JUN-1,R2,(BC)GTTTAAGAGCTAAGCTGGAA,AGCAGGGCTCTCCTCCCGG,CRISPR Guide Capture,ENSG00000177606,JUN
JUN-2,JUN-2,R2,(BC)GTTTAAGAGCTAAGCTGGAA,TGTGGCTGAAGCAGCGAGG,CRISPR Guide Capture,ENSG00000177606,JUN
10X fastq filename
As SCREE alignment part is based on cellranger, names of input fastq file must in the same format:
SCREE analysis part
The second part of SCREE is an integrated R package, using the output of SCREE alignment part as input.
sessionInfo()R version 4.0.2 (2020-06-22)
Platform: x86_64-apple-darwin17.0 (64-bit)
Running under: macOS Catalina 10.15.7
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRblas.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] workflowr_1.6.2
loaded via a namespace (and not attached):
[1] Rcpp_1.0.7 whisker_0.4 knitr_1.33 magrittr_2.0.1
[5] R6_2.5.0 rlang_0.4.11 fansi_0.5.0 stringr_1.4.0
[9] tools_4.0.2 xfun_0.25 utf8_1.2.2 git2r_0.28.0
[13] jquerylib_0.1.4 htmltools_0.5.1.1 ellipsis_0.3.2 rprojroot_2.0.2
[17] yaml_2.2.1 digest_0.6.27 tibble_3.1.3 lifecycle_1.0.0
[21] crayon_1.4.1 later_1.2.0 sass_0.4.0 vctrs_0.3.8
[25] promises_1.2.0.1 fs_1.5.0 glue_1.4.2 evaluate_0.14
[29] rmarkdown_2.10 stringi_1.7.3 bslib_0.2.5.1 compiler_4.0.2
[33] pillar_1.6.2 jsonlite_1.7.2 httpuv_1.6.1 pkgconfig_2.0.3